In the past few years, extremely drug resistant strains of TB have arisen that can’t be eliminated by any drugs, so new strategies for attacking TB are urgently needed.
Now, researchers at Albert Einstein College of Medicine of Yeshiva University have found two novel ways of killing the bacteria that cause tuberculosis.
In searching for a new Achilles’ heel for
M. tuberculosis,
Dr. Jacobs and colleagues focused on an enzyme called
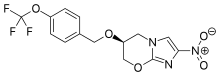
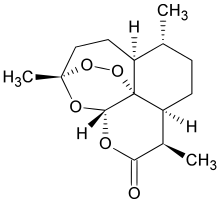
GlgE. Previous research had suggested that GlgE might be essential for the growth of TB bacteria. (building polysaccharides) GlgE would also be an excellent drug target because there are no enzymes similar to it in humans or in the bacteria of the human gut.
Using genetic and biochemical approaches, William Jacobs and colleagues identified four enzymes involved in a pathway that converts a naturally-occurring sugar compound into polysaccharides called alpha-glucans. The scientists found that inactivating one of these enzymes, TreS, was not lethal to the bacteria, indicating that this pathway is not required for growth.
However, inactivating GlgE was lethal, causing the buildup of toxic levels of the enzyme's sugar substrate, maltose-1-phosphate. In addition, the scientists found that the combined inactivation of TreS and an enzyme for an alternate alpha-glucan biosynthetic pathway was lethal, highlighting the important roles of alpha-glucan's in M. tuberculosis growth.
Sure enough, when the researchers inhibited GlgE, the bacteria underwent "suicidal self-poisoning": a sugar called maltose 1-phosphate accumulated to toxic levels that damaged bacterial DNA, causing the death of TB bacteria grown in Petri dishes as well as in infected mice.
The researchers discovered a second way of killing TB after observing a crucial connection between their novel alpha glucan pathway and a second pathway that also synthesizes alpha glucans.
When the researchers knocked out one of the other enzymes in their novel pathway, the pathway's shutdown didn't kill the bacteria; similarly, inactivating an enzyme called Rv3032 in the second alpha glucan pathway failed to kill the microbes. But inactivating both of those enzymes caused what the researchers term synthetic lethality: two inactivations that separately were nonlethal but together cause bacterial death.
Though the biological role of the GlgE pathway remains to be elucidated, GlgE and the alpha-glucan pathways more generally, are possible drug targets that can now be tested in in vivo models of tuberculosis infection....
"The bacteria that cause TB need to synthesize alpha glucans," notes Dr. Jacobs. "And from the bacterial point of view, you can't knock out both of these alpha glucan pathways simultaneously or you're dead. So if we were to make drugs against GlgE and Rv3032, the combination would be extremely potent. And since TB bacteria need both of those alpha glucan pathways to live, it's very unlikely that this combination therapy would leave behind surviving bacteria that could develop into resistant strains."
Ref : http://www.nature.com/nchembio/journal/vaop/ncurrent/pdf/nchembio.340.pdf